Stefano Berto, PhD
My laboratory was established at MUSC in 2021, and as a new and early-stage investigator, I aim to elucidate the molecular signaling pathways involved in neurodevelopmental disorders (NDDs) and brain evolution. Our research focuses on identifying novel genes and signaling pathways implicated in brain disorders, utilizing studies on transcription and splicing factors, as well as employing comparative genomics and other computational approaches. One of the key methodologies my lab will employ is the use of human and non-human primate iPSCs, combined with multiomics at the single-cell level, to investigate the precise developmental dynamics of cell-type-specific maturation and differentiation. Additionally, this approach will enable us to study genes associated with human complex traits and NDDs. Furthermore, I hold the position of Director of the Bioinformatics Core, where I currently oversee five bioinformaticians working on genomics of cancer and other diseases.
Open Positions
We are actively looking for motivated graduate students and post-doc candidates. Please write to us with a cover letter and resume if you are interested in what we do.
Scientific directions of the Berto Lab
Our laboratory aims to understand the cellular and molecular basis of human brain development and neurodevelopmental disorders. By combining human in-vitro models such as 2D neurons and 3D organoid cultures with advanced genomic approaches, we aim to study genes involved in autism and neurodevelopmental disorders (NDD).
In particular, we are deeply committed to study the role of CHAMP1, a high-risk NDD gene, in human brain development. By using patient-derived iPSC combined with single cell multiomics and electrophysiology, we are characterizing the effect of different mutations in developing human neurons and cortical organoids. Our ultimate goal is to develop new treatments for this rare human neurodevelopmental syndrome.
The other project in our lab focuses on the function of the NDD risk gene, IL1RAPL2, in developing human neurons and brain connectivity. We use human iPSC-derived excitatory neurons where we knocked out IL1RAPL2 and ASD-like and memory behaviors using a new mouse model. We hypothesize that IL1RAPL2 plays a key role in neurodevelopmental disorders and memory phenotypes by regulating synapse development on cortical excitatory neurons
Moreover, by using single cell multiomic approaches, we are interested in the evolution of the human brain. We are particularly interested in oligodendrocytes evolution, development, and how they are implicated in neuropsychiatric disorders. We have previously revealed that oligodendrocytes have undergone an increased acceleration on human lineage compared with neurons and oligodendrocyte human specific genes are enriched for variants associated with neuropsychiatric disorders. Moreover, oligodendrocytes showed differences between healthy and schizophrenia post-mortem brain tissue. Overall, these findings underscored the importance of oligodendrocytes in human brain evolution and disease. We will take advantage post-mortem white matter brain tissue coupled with single cell multiomic approaches to further answer questions on oligodendrocytes evolution and development.
Research Interest
Neurogenomics of the Human Brain Evolution

Role of CHAMP1 in brain development
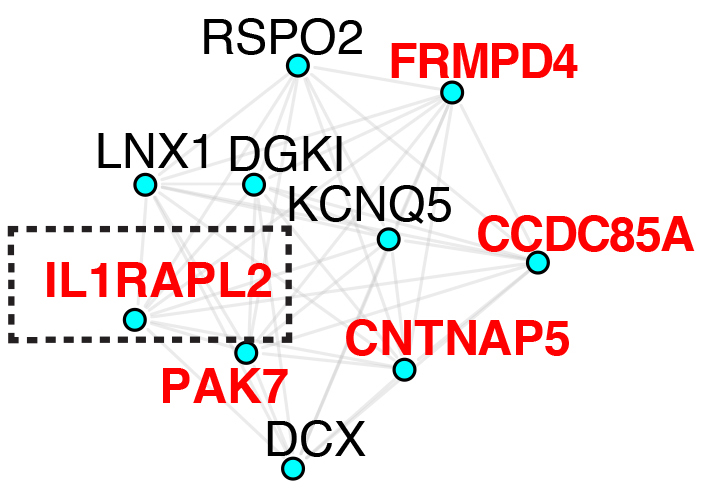
Role of IL1RAPL2 in neuronal development and function

